Born January 27 (February 8, New Style), 1834, Tobolsk, Siberia, Russian Empire.
Died January 20 (February 2), 1907, St. Petersburg, Russia)
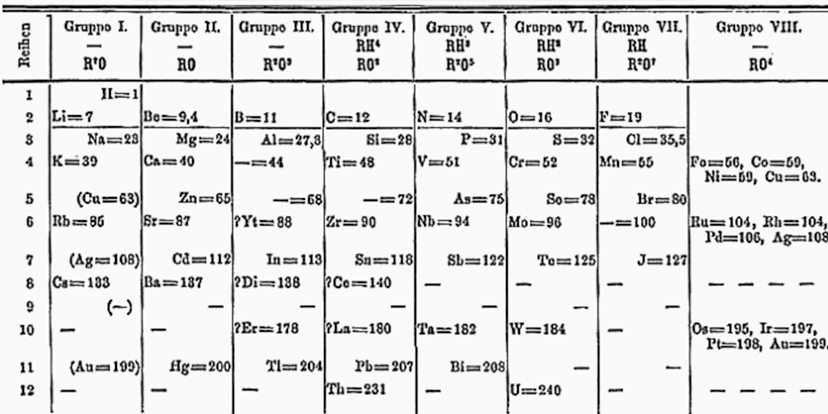
Dmitri Ivanovich Mendeleev was a Russian chemist and inventor. He is best known for formulating the Periodic Law and creating a version of the periodic table of elements
He used the Periodic Law not only to correct the then-accepted properties of some known elements, such as the valence and atomic weight of uranium, but also to predict the properties of three elements that were yet to be discovered (germanium, gallium and scandium).