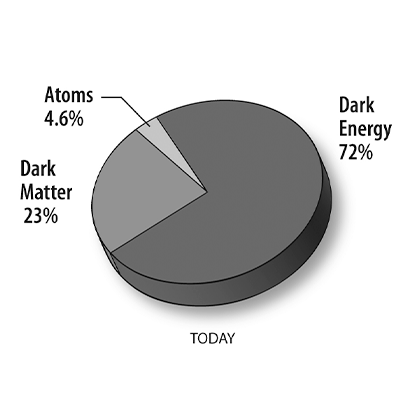
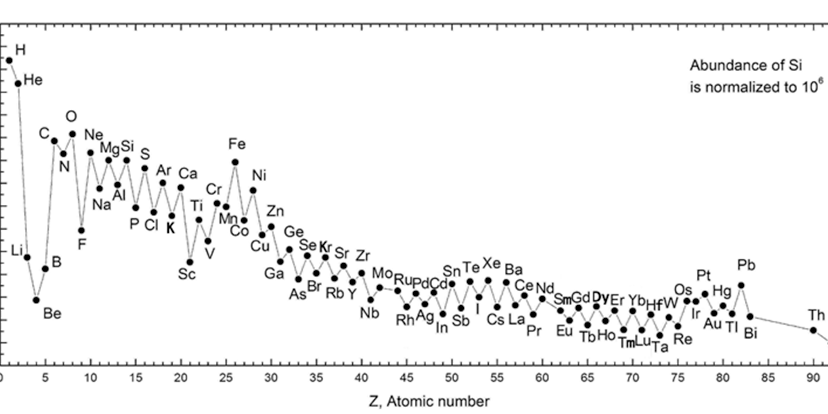
The 94 naturally occurring elements were produced by at least four classes of astrophysical process. Most of the hydrogen, helium and a very small quantity of lithium were produced in the first few minutes of the Big Bang. This Big Bang nucleosynthesis happened only once; the other processes are ongoing. Nuclear fusion inside stars produces elements through stellar nucleosynthesis, including all elements from carbon to iron in atomic number. Elements higher in atomic number than iron, including heavy elements like uranium and plutonium, are produced by various forms of explosive nucleosynthesis in supernovae and neutron star mergers. The light elements lithium, beryllium and boron are produced mostly through cosmic ray spallation (fragmentation induced by cosmic rays) of carbon, nitrogen, and oxygen.